Reports and Dossier
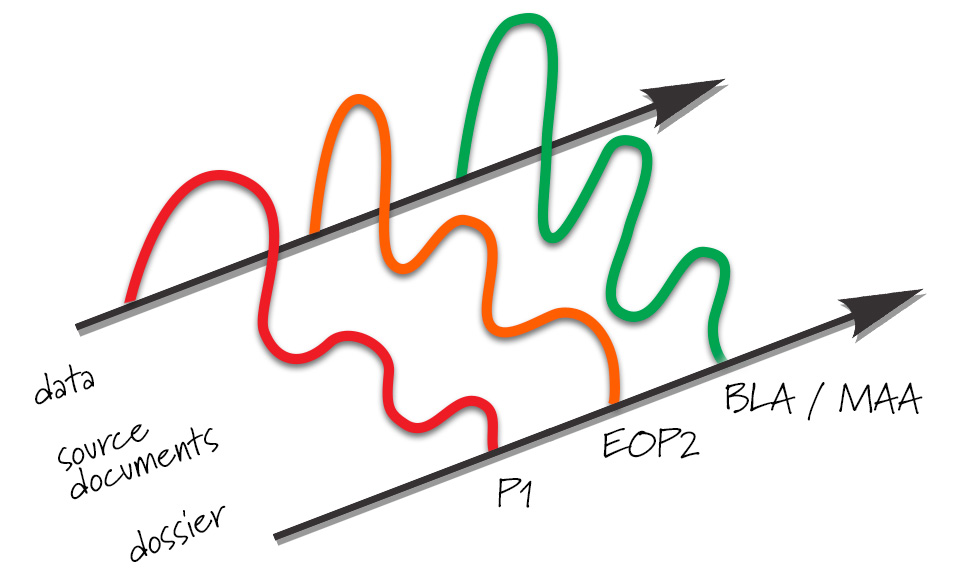
Levels in data and documentation evolve with the product development timeline. As development proceeds, data will increase. At phase 1, source documents and the dossier are in early stages. Source documentation should keep pace with the increase in data during product development, and a good set of source documents is preferable by End-of-Phase 2. The dossier may grow more slowly, but there is a surge in dossier content for the final license applications (BLA/MAA).
Good documentation is the foundation of product development.
- Dossier: Good communication and technical writing is the cornerstone of a regulatory submission.
- Source documents: Good practice in dossier preparation is to ensure that the contents is archived in source documents. Great source documents should include a summary section that is dossier-friendly, linking the regulatory message through the scientific assessment to the technical source data.
REGEXBIO can assist in the design, technical writing or review of source documents or dossier sections.